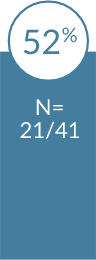Significantly higher patient satisfaction with
In a head-to-head trial with 419 pregnant women, patients were more satisfied with
Patients who received
- Better sleep (P=0.01)
- More relaxing time (P=0.001)
- Ability to perform daily activities (P=0.001)